

|
|
| Thermodynamics and Propulsion | |
6.9 Muddiest Points on Chapter 6
MP 6..1
So, do we lose the capability to do work when we have an
irreversible process and entropy increases?
Absolutely. We will see this in a more general fashion very soon. The idea of lost work is one way to view what ``entropy is all about.''
MP 6..2
Why do we study cycles starting with the Carnot cycle? Is it
because it is easier to work with?
Carnot cycles are the best we can do in terms of efficiency. We use the Carnot cycle as a standard against which all other cycles are compared. We will see in class that we can break down a general cycle into many small Carnot cycles. Doing this we can gain insight in which direction the design of efficient cycles should go.
MP 6..3
How does one interpret h-s diagrams?
I find
MP 6..4
Is it always OK to ``switch'' T-s and h-s diagrams?
No! This is only permissible for perfect gases with constant
specific heats. We will see, when we examine cycles with
liquid-vapor mixtures, that the
MP 6..5
What is the best way to become comfortable with T-s
diagrams?
I think working with these diagrams may be the most useful way to
achieve this objective. In doing this, the utility of using these
coordinates (or
MP 6..6
What is a reversible adiabat physically?
Let's pick an example process involving a chamber filled with a
compressible gas and a piston. We assume that the chamber is
insulated (so no heat transfer to or from the chamber) and the
process is thus adiabatic. Let us also assume that the piston is
ideal, such that there is no friction between the walls of the
chamber and the piston. The gas is at some temperature
MP 6..7
If there is an ideal efficiency for all cycles, is there a
maximum work or maximum power for all cycles?
Yes. As with the Brayton cycle example, we could find the maximum as a function of the appropriate design parameters.
MP 6..8
Do we ever see an absolute variable for entropy? So far, we
have worked with deltas only.
It is probably too strong a statement to say that for ``us'' the
changes in entropy are what matters, but this has been my experience
for the type of problems aerospace engineers work on. Some values of
absolute entropy are given in Table A.8 in SB&VW. We will also see,
in the lectures on Rankine cycles, that the entropy of liquid water
at a temperature of
MP 6..9
I am confused as to
Both of these are true and both can always be used. The first is the
definition of entropy. The second is a statement of how the entropy
behaves. Section 6.5 attempts to make the
relationship clearer through the development of the equality
MP 6..10
For irreversible processes, how can we calculate
We need to define a reversible process between the two states in order to calculate the entropy (see MP 6.9). See VN Chapter 5 (especially) for discussion of entropy or Section 6.5. If you are still in difficulty, come and see me.
MP 6..11
Is
No. Entropy is a function of state (see Gibbs) and thus
MP 6..12
Are
Yes. We have an irreversible process from state 1 to state 2. We then used a reversible process to restore the initial state 1 (we had to do work on the system and extract heat from the system).
MP 6..13
Why do we find stagnation enthalpy if the velocity never
equals zero in the flow?
The stagnation enthalpy (or stagnation temperature) is a useful
reference quantity. Unlike the static temperature it does not vary
along a streamline in an adiabatic flow, even if irreversible. It
was thus the natural reference temperature to use in describing the
throttling process. In addition, changes in stagnation enthalpy (or
stagnation temperature) are direct representations of the shaft work
or heat associated with a fluid component. The static enthalpy is
not, unless we assume that changes in
MP 6..14
Why does
Because for a steady adiabatic flow with no shaft work done the
Steady Flow Energy Equation yields constant stagnation enthalpy even
though the flow processes might not be reversible (see notes). For a
perfect gas with constant specific heats,
MP 6..15
How do you tell the difference between shaft work/power and
flow work in a turbine, both conceptually and mathematically?
Let us look at the expansion of a flow through a turbine using both
the control mass approach and the control volume approach. Using the
control mass approach we can model the situation by tracking
When entering the turbine, the fluid has to push the surroundings out of the way to make room for itself (it has volume
The right hand side of the above equation is the change in enthalpy
We can also solve this problem by using the 1st law in general form (control volume approach):
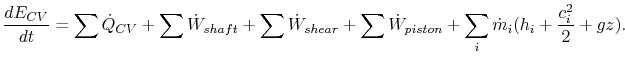
Note that in this form the flow work is buried in
We obtain the same result as before:
MP 6..16
Is the entropy change in the equations two lines above the
total entropy change? If so, why does it say
The entropy change in question is the entropy change due to the heat produced by friction only.
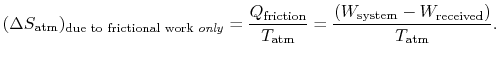
MP 6..17
Why does
When we wrote this equality, we were considering a system that was returned to its original state, so that there were no changes in any of the system properties. All evidence of irreversibility thus resides in the surroundings.
MP 6..18
In discussing the terms ``closed system'' and ``isolated
system,'' can you assume that you are discussing a cycle or not?
The terms closed system and isolated system have no connection to whether we are discussing a cycle or not. They are attributes of a system (any system), whether undergoing cyclic behavior, one-way transitions, or just sitting there.
MP 6..19
Does a cycle process have to have
Entropy is a state function. If the process is cyclic, initial and
final states are the same. So, for a cyclic process,
MP 6..20
In a real heat engine, with friction and losses, why is
The change in entropy during a real cycle is zero because we are
considering a complete cycle (returning to initial state) and
entropy is a function of state (holds for both ideal and real
cycles!). Thus if we integrate
We will see this better using the
MP 6..21
Is it safe to say that entropy is the
tendency for a system to go into disorder?
Entropy can be given this interpretation from a statistical perspective, and this provides a different and insightful view of this property. At the level in which we have engaged the concept, however, we focus on the macroscopic properties of systems, and there is no need to address the idea of order and disorder; as we will see, entropy is connected to the loss of our ability to do work, and that is sufficient to make it a concept of great utility for the evaluation and design of engineering systems. We will look at this in a later lecture. If you are interested in pursuing this, places to start might be Great Ideas in Physics by Lightman (paperback book by an MIT professor), or Modern Thermodynamics by Kondepudi and Prigogine. UnifiedTP |